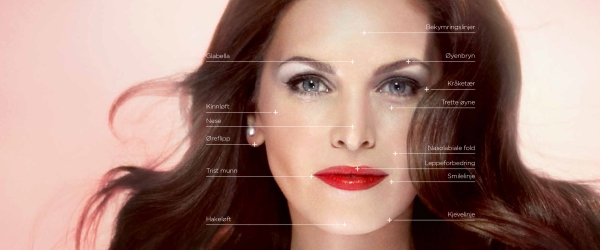
Restylane Vital – Skin Booster
Launched in the UK at the beginning of 2005, RESTYLANE® Vital is a new NASHA gel manufactured by Q-Med (now part of Galderma), the maker of the popular dermal fillers RESTYLANE®, RESTYLANE® Touch, RESTYLANE® SubQ and RESTYLANE PERLANE® ; aimed at a whole new approach to skin rejuvenation.
It is targeted at a broad range of treatment areas including the face, neck, décolletage (the area between the neck and breasts) and hands; designed to replenish the hyaluronic acid lost through ageing, hence hydrating the skin and improving its elasticity and tone.
Why is it best?
- The recommended injection technique is the micro-puncture technique that involves injecting very small amounts of RESTYLANE® Vital into the deep dermis, about 1- 2cm apart. Once injected, the NASHA gel flows smoothly and distributes evenly in the skin.
- The natural hydrating properties of RESTYLANE® Vital also make it well suited to all skin types, whether skin is dry or oily.
- The RESTYLANE® range of products has a proven track record of safety with more than two million treatments across 70 countries, and FDA approval. RESTYLANE® Vital also obtained European CE certification in 2004 for the indication for hydration of the skin by injections.
Treatment Video
Coming Soon
Important Notes
Generic name
Non-Animal Stabilized Hyaluronic Acid (NASHA) gel.
What do Restylane Skinboosters contain?
RESTYLANE® Skinboosters are made from Q-Med’s patented NASHA gel, with no extra additives. They also come with or without 0.3% lidocaine as a local anaesthetic.
How is Restylane® Vital made?
By bacterial fermentation from streptococci bacteria.
Is a skin test required before Restylane® Skinbooster treatment?
No allergy test needed.
Is Restylane® Vital temporary or permanent?
Hyaluronic acid is completely broken down within the skin over a period of months, eventually leaving no trace of the filler.
Licensed status
Medical device.
Should be used by
Restylane® Skinboosters should be used by trained members of the medical profession only.
Not to be used in
Individuals with a known hypersensitivity to hyaluronic acid.
RESTYLANE® Skinboosters should not be used in or near areas where there is active skin disease, inflammation or related conditions.
RESTYLANE® Skinboosters should not be injected into an area where a permanent implant has been placed.
Results
Reported side effects of Restylane® Skinboosters
After the injection of RESTYLANE® Vital, some common injection related reactions might occur. These reactions include transient erythema (redness), swelling, pain, itching, discolouration or tenderness at the injection site. Typically resolution is spontaneous, within one or two days after injection into the skin.
Additionally, temporary palpable lumpiness has been noted after injection in some patients.
Localised reactions thought to be of a hypersensitivity nature have been reported in about 1 in every 10,000 treated patients. These have consisted of swelling at the injection. Redness, tenderness and rarely acneform papules may occur.
The reactions have started either shortly after injection or after a delay of 2-4 weeks and have generally been described as mild to moderate and self-limiting, with an average duration of 2 weeks.
In addition, rare cases (less than I in 15,000 treatments) of granuloma formation, superficial necrosis and urticaria have been reported.
Downtime
This depends upon your age and lifestyle as well as the correct placement of the product by a practitioner.
Generally, two to three treatments every fourth week are recommended initially, followed with maintenance treatments approximately every four to six months for Vital and Vital Light.
RESTYLANE® Lip Refresh results are expected to last for up to 6 months.
Cost
1 treatment – £199
3 treatments – £499
